Rehabilitation Device for Post-Knee Replacement
Type: Design Challenge | Role: Lead Mechanical Concept & Biomechanics Designer | Status: Preliminary Concept
Challenge Host: Jack McDonald (48-Hour Biomedical Device Innovation Challenge)
Overview
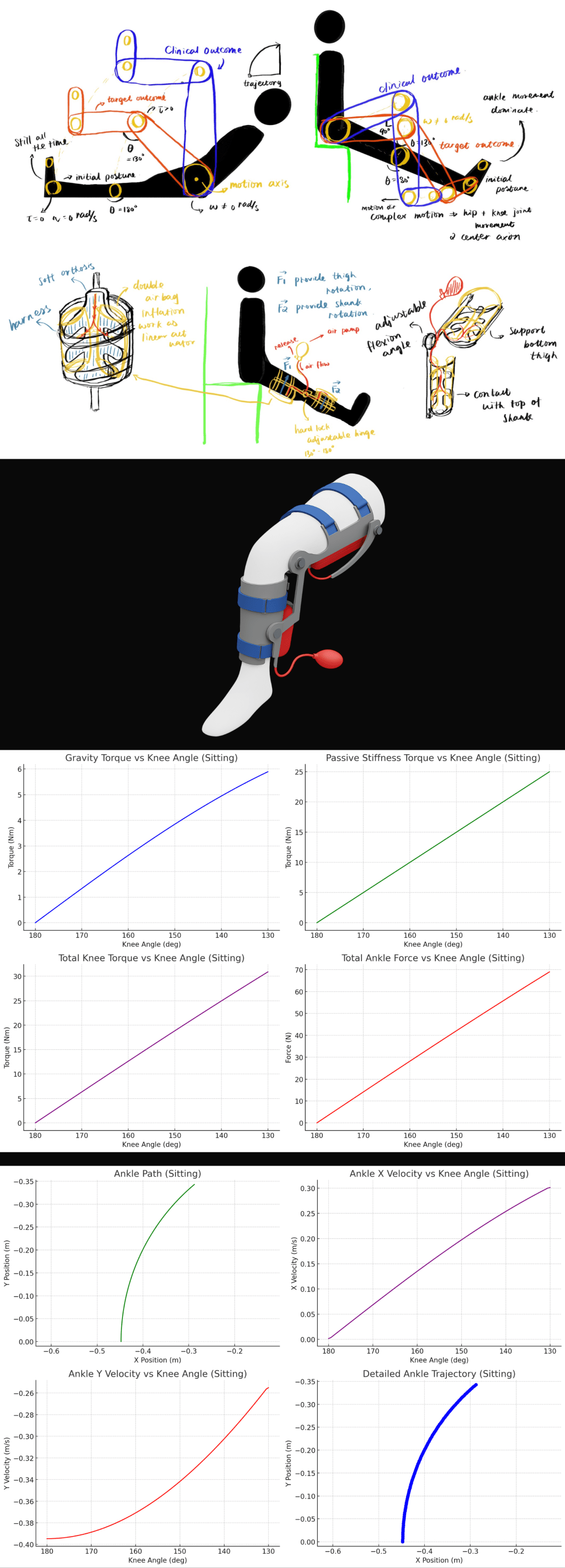
This project explores non-motorized rehabilitation methods for post-knee replacement patients. In response to a 48-hour challenge, a passive device was developed to promote safe and independent knee flexion-extension motion. The design integrates pneumatic cushions and an ergonomic orthotic structure to assist home recovery without requiring supervision or electrical power.
Design Objectives
- Enable passive knee flexion-extension from 180° to 130° to simulate CPM therapy outcomes
- Eliminate powered actuation for a Class I medical device classification
- Ensure ergonomic conformity and comfort during extended use in relaxed settings (e.g., watching TV)
- Design modular and lightweight components to ease fabrication and assembly
- Support safe user-adjustable motion with mechanical hard stops
Key Features
Form Factor:
- Inflatable cushion-actuated system with dual-segment support structure
- Integrated orthosis-inspired frame for stable fit on thigh and shank
- Adjustable flexion lock at the knee joint (set to 130° max angle)
Core Mechanism:
- Four angled air bladders (2 below thigh, 2 above shank) simulate linear actuators
- Manual air pump controls with optional valve isolation for independent segment motion
- Hard-stop hinge with mechanical lock and passive angle limitation
Materials & Structure:
- Lightweight polycarbonate for frame and joint structures
- Medical-grade TPU for air cushions
- Soft foam liners and Velcro harnesses for skin comfort and secure positioning
Technical Contributions
- Conducted full-body kinematic and kinetic simulation using anthropometric data (75th percentile male/female)
- Identified passive joint resistance and target actuator force profiles for 130° ROM
- Generated engineering specification tables from biomechanical analysis
- Developed multiple functional diagrams and ergonomic concept sketches
- Iteratively refined CAD mockups with pneumatic and structural system integration
Intellectual Property Note
The device design and joint modeling strategies are original and documented for academic use. The actuation method and orthosis structure reflect general mechanical principles and are intended for prototyping exploration. All conceptual work remains non-commercial and subject to further validation.